Select Chapter Topics:
Ratio of the times required to reach 99% completion (\(\mathrm { t_{99} }\)) and 90% completion (\(\mathrm { t_{90} }\)) for a first-order reaction is:
1.
2
2.
4
3.
0
4.
5
Subtopic: First Order Reaction Kinetics |
82%
Level 1: 80%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Given two first-order reactions, with the ratio of their half-lives:
\(\frac{t_{1 / 2}^1}{t_{1 / 2}^2}=\frac{2}{5} .\)Calculate the ratio of their fractional half-lives: \(\frac{t_{2 / 3}^1}{t_{4 / 5}^2}=\)?
1. 0.273
2. 0.468
3. 0.318
4. 2.55
\(\frac{t_{1 / 2}^1}{t_{1 / 2}^2}=\frac{2}{5} .\)Calculate the ratio of their fractional half-lives: \(\frac{t_{2 / 3}^1}{t_{4 / 5}^2}=\)?
1. 0.273
2. 0.468
3. 0.318
4. 2.55
Subtopic: First Order Reaction Kinetics |
67%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The activation energy of one of the reactions in a biochemical process is \(\mathrm{532611~ J~ mol^{–1} .}\) When the temperature falls from \(\mathrm{310~ K}\) to \(\mathrm{300~ K,}\) the change in rate constant observed is \(\mathrm{k_{300} = x × 10^{–3} k_{310}.}\)
The value of \(\mathrm{x}\) is:
[Given: ln\(\mathrm{10 = 2.3, R = 8.3 ~JK^{–1} mol^{–1}}\) ]
The value of \(\mathrm{x}\) is:
[Given: ln\(\mathrm{10 = 2.3, R = 8.3 ~JK^{–1} mol^{–1}}\) ]
| 1. | 1 | 2. | 5 |
| 3. | 2 | 4. | 8 |
Subtopic: Arrhenius Equation |
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
For a reaction, \(\mathrm{N}_2 \mathrm{O}_{5(\mathrm{~g})} \rightarrow 2 \mathrm{NO}_{2(\mathrm{~g})}+\frac{1}{2} \mathrm{O}_{2(\mathrm{~g})}\) in a constant volume container, no products were present initially. The final pressure of the system when 50% of the reaction gets completed is:
1. 7/2 times of initial pressure
2. 5 times of initial pressure
3. 5/2 times of initial pressure
4. 7/4 times of initial pressure
1. 7/2 times of initial pressure
2. 5 times of initial pressure
3. 5/2 times of initial pressure
4. 7/4 times of initial pressure
Subtopic: First Order Reaction Kinetics |
71%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given elementary reaction:
\(\mathrm{A}(\mathrm{~g})+\mathrm{B}(\mathrm{~g}) \rightarrow \mathrm{C}(\mathrm{~g})+\mathrm{D}(\mathrm{~g})\)
If the volume of the reaction mixture is suddenly reduced to \(\frac{1}{3}\) of its initial volume, the reaction rate will become ‘x’ times the original reaction rate. The value of x is:
\(\mathrm{A}(\mathrm{~g})+\mathrm{B}(\mathrm{~g}) \rightarrow \mathrm{C}(\mathrm{~g})+\mathrm{D}(\mathrm{~g})\)
If the volume of the reaction mixture is suddenly reduced to \(\frac{1}{3}\) of its initial volume, the reaction rate will become ‘x’ times the original reaction rate. The value of x is:
| 1. | \(\dfrac{1}{9}\) | 2. | 9 |
| 3. | \(\dfrac{1}{3}\) | 4. | 3 |
Subtopic: Definition, Rate Constant, Rate Law |
57%
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Given below are two graphs:
In the light of the above two graphs, choose the correct answer from the options given below :
| Graph I: | 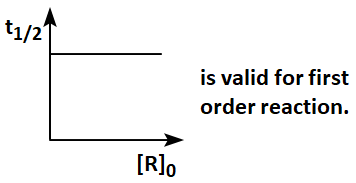 |
| Graph II: | 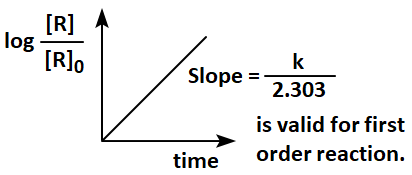 |
| 1. | Both graph I and graph II are not correct. |
| 2. | Graph I is not correct but graph II is correct. |
| 3. | Both graph I and graph II are correct. |
| 4. | Graph I is correct, but graph II is not correct. |
Subtopic: First Order Reaction Kinetics |
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
For a first-order reaction, a half-life \((t_{1/2}) \) is 50 min. The time \(t_{3/4} \) (in minutes) of the reaction is:
1. 75
2. 100
3. 125
4. 50
1. 75
2. 100
3. 125
4. 50
Subtopic: First Order Reaction Kinetics |
79%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
A reaction follows 1st order kinetics with rate constant (k) = 20 min-1. Calculate the time required to reach the concentration to 1/32 times of initial concentration.
1. 0.17325 min
2. 1.7325 min
3. 17.325min
4. 173.25 min
1. 0.17325 min
2. 1.7325 min
3. 17.325min
4. 173.25 min
Subtopic: First Order Reaction Kinetics |
84%
Level 1: 80%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
For first-order kinetic rate constant \(2.011 \times 10^{-3} \mathrm{sec}^{-1} \). The time taken for the decomposition of the substance from 7g to 2g will be:
(Use log7 = 0.845 and log2 = 0.301)
1. 647 sec
2. 598 sec
3. 623 sec
4. 604 sec
(Use log7 = 0.845 and log2 = 0.301)
1. 647 sec
2. 598 sec
3. 623 sec
4. 604 sec
Subtopic: First Order Reaction Kinetics |
62%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following first-order reactions:
\(A \rightarrow C ; t_{1/2}=15 \mathrm{~min}, B \rightarrow D ; t_{1/2}=5 \mathrm{~min} \)
The initial concentrations of A and B are 1 molar and 8 molar respectively. The time when the concentration of A and B becomes equal is ‘X’ minutes. The value of 2X is:
1. 40 min
2. 50 min
3. 45 min
4. 54 min
\(A \rightarrow C ; t_{1/2}=15 \mathrm{~min}, B \rightarrow D ; t_{1/2}=5 \mathrm{~min} \)
The initial concentrations of A and B are 1 molar and 8 molar respectively. The time when the concentration of A and B becomes equal is ‘X’ minutes. The value of 2X is:
1. 40 min
2. 50 min
3. 45 min
4. 54 min
Subtopic: First Order Reaction Kinetics |
53%
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.






